|
by Marta Siek
Proteins, with their complex structures and diverse functions, have long served as a source of inspiration for the design of functional nanomaterials. The specificity of their interactions and the precision of their actions, as seen in enzymes and chaperones, have motivated scientists to create protein-mimicking nanomaterials for specific tasks. However, while much attention has been given to the active sites and surfaces of protein-protein interactions, the outer surfaces that determine a protein's interactions with the external environment, particularly its biocompatibility, have been regrettably neglected. Scientists from the Center for Algorithmic and Robotized Synthesis, Institute for Basic Science in Ulsan, South Korea, decided to rectify this oversight and performed a statistical analysis of 15000 structures of proteins, testing their shapes, hydrophobicity, ability to form hydrogen bonds, charge, and correlations thereof. They compiled and quantified the features characteristic of all proteins as well as of specific groups, e.g., secreted or nuclear proteins or mammalian versus bacterial proteins. Next, the group of bio-active nanoparticles was subjected to the same analysis as proteins, and a comparison between them was published in the journal Small Structures (2023 IF 13.9). As a case study, the authors chose gold nanoparticles covered with a mixture of positively and negatively charged alkanethiols, which already showed unparalleled bio-mimicking behavior, like protein-like specific catalysis, Gram-discerning toxicity towards bacteria, or very highly selective cytotoxicity towards cancer cells. Those nanoparticles, so-called mixed-charge nanoparticles, MCNPs, resemble proteins not only in their sizes (<10 nm of hydrodynamic diameter) or globular shapes, but also in the surface topology patches of charge that are separated by hydrophobic canyons. "We shared the code for our analysis so everyone can see to what extent their nanomaterials resemble proteins. Compilation of such analyses should give us a handbook of surface properties and help in the more conscious design of nanomaterials. Most interesting right now seem to be interactions of nanomaterials with proteins and with other nanoobjects, formation of suprastructures that can change the immune response or metabolic stability of designed materials" - said the first author of the analysis, Dr. John McBride. 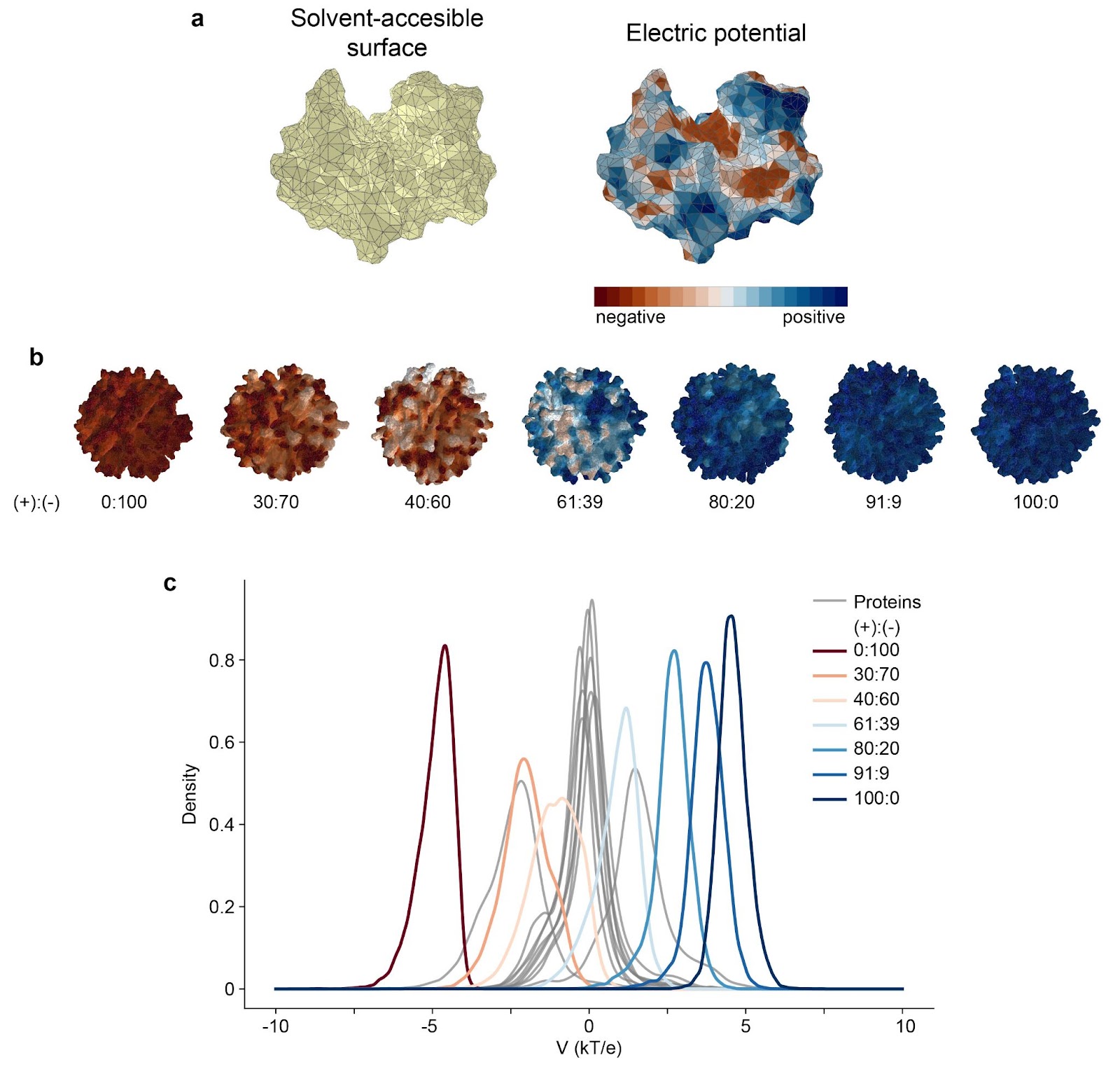
Figure 1. Exemplary statistical analysis of the distribution of electric potential on the protein (a, here hen lysozyme, PDB 194L) and on MCNPs with different ratios of positively and negatively charged ligands in the shell (b) shows that compared with proteins MCNPs allow for almost unrestricted tuning of the overall surface potential just by the modification of surface chemistry. Statistical Survey of Chemical and Geometric Patterns on Protein Surfaces as a Blueprint for Protein-Mimicking Nanoparticles John M. McBride,* Aleksei Koshevarnikov, Marta Siek, Bartosz A. Grzybowski* & Tsvi Tlusty*. Small Structures, 2400086 (2024). Check out the original article here!
|